Determination of water content in animal and vegetable fats and oils
Release Time
2025-09-11 10:46
The REX KFT-40VC Karl Fisher Titrator allows quick and simple determinations of the water content of animal and vegetable fats and oils.
Fats and oils can be found naturally in a wide range of animal and plant-based sources. They serve an important part of a balanced and healthy diet as they provide energy, support growth and development, provide the essential fatty acids and boost the immune system.
Oils or fats which are obtained solely from plant or vegetable sources are vegetable oils. In the solid form, they are called fats, while in the liquid form, they are called oils. Their molecular structure is composed of large molecules called triglycerides, monoglycerides and diglycerides and lipids. You can extract oil by crushing the seeds or fruit of the plant. Certain oils like herb oils are extracted from the leaves or the plant itself.
Fat or grease which is obtained from animal sources and materials like tissues and bones. Some examples are: Lard – pig fat obtained from various parts of the pig’s body,Poultry fat – fat obtained from chicken, duck or goose, Tallow – beef or lamb fat, Fish oil.
Moisture content is a key quality indicator for animal and vegetable fats and oils.

1. Standard Reference
ISO 8534:2017 Surface active agents — Animal and vegetable fats and oils — Determination of water content — Karl Fischer method (pyridine free)
GB/T 26626-2011 Animal and vegetable fats and oils-Determination of water content Karl Fischer method (pyridine free)
2. The Principle
ISO 8534:2017: Dissolved fat is titrated against an iodine solution and sulfur dioxide (SO2) is oxidized by iodine in the presence of water. In principle, the chemical reaction in Formula takes place:
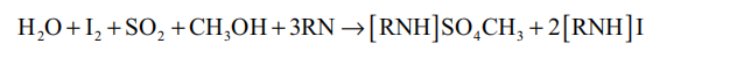
The alcohol reacts with SO2 and a nitrogenous base (RN) to form an intermediate alkylsulfite salt, which is then oxidized by iodine to an alkylsulfate salt. This oxidation reaction consumes water contained in the sample. The end point is monitored potentiometrically.
The determination of water is conducted by adjusting the sample size to have between 1 mg and 100 mg water for the volumetric titration (the main body of this document) and between 10 µg and 10 mg for the coulometric titration (Annex B) using Karl Fischer instruments and reagents which have been validated with standard water solutions over the necessary range. For the volumetric determination, a minimum amount of 0.5 ml Karl Fischer reagent shall be used for the titration.
3. Recommended Configuration
KFT-40VC Karl Fisher Titrator
- Support Volumetric Karl Fischer Titration
- Support Auto Titrate Mode and Titer Measure Mode.
- Features of auto-filling, auto-purging and auto-mixing of the reagents ensure safe handling of Karl Fischer chemicals.
- Automatic/manual background drift correction ensures accurate results.
- Selectable units including mg, mg/L, %, ppm, etc.
- Settable parameters, including measurement unit, polarization current, stirring rate, titration rate, stop volume, endpoint potential, stop criterion, etc.
- Store up to 200 titration data sets (GLP-compliant).

4. Volumetric Karl Fischer Titration Procedure:
Titre
- The titre shall be determined daily for each bottle of titrant.
- Add 20 ml to 40 ml of working solvent to the titration vessel. The solvent should cover the platinum electrodes.
- Titrate the vessel to a stable dry end point. CAUTION — Take care not to overtitrate.
- Determine the titre of the titrant using the water standard and a syringe. Sample mass is determined by difference.
- Start the titration and record the titre when a stable end point is reached. Some instruments may require calculation of titre from the displayed percentage of water.
- Average a minimum of three titre determinations. Record the arithmetic average.
- Update the instrument titre value with the new setting.
Test portion
- By means of a syringe, weigh and introduce a portion of the sample into the instrument using the target masses.At least 0.5 ml Karl Fischer reagent shall be used for the titration.
Determination
- Add 20 ml to 40 ml working solvent (5.1) to the titration vessel. The solvent should cover theplatinum electrodes.
- Titrate the vessel to a stable dry end point. CAUTION -Take care not to overtitrate.
- Weigh and introduce the test portion into the instrument according to 9.2.
- Record the moisture content of the test portion when a stable dry end point is reached.
- Up to six test portions may be assayed before replacing and pre-titrating the working solvent.
5. Titration Setting
- Volumetric Karl Fischer Titration
- Method: KF titer determination
- Method: Auto Titrate

PRECISION PERFECT
Contact Us
-
Tel:0086-021-39506429/39506392
Mail:info@lei-ci.com
-
Fax: 0086-021-39506398


 Contact
Contact E-mail
E-mail
